Universal Chalcidoidea Database
Notes on families
Perilampidae
Main diagnostic characters
1. Prepectus in same plane as, and fused with, pronotum (80%)
2. Pronotum in dorsal view with sides more or less parallel and medially clearly visible behind head (100%)
3. Fore wing with marginal vein moderately long and postmarginal and stigmal veins short (100%)
4. Moderately large, about 1.3-5.5mm in length (100%)
Included taxa
The family currently includes 15 genera and 277 species placed in 3 subfamilies as follows: Chrysolampinae (6/63), Perilampinae
(6/202), Philomidinae (2/11), unplaced (1/1).
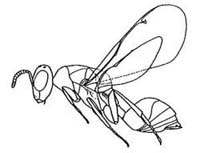 |
 |
| Chrysolampinae (Austrotoxeuma kuscheli) |
Perilampinae (Perilampus sp.) |
Biology
Many species of the larger subfamily, the Perilampinae, are hyperparasitic, developing on tachinid and ichneumonoid primary parasitoids of Lepidoptera and Symphyta (Clausen, 1940). Some species (eg the North American Perilampus fulvicornis) seem to be obligate hyperparasitoids, but others (eg P. hyalinus) appear to be facultative and capable of developing equally well on diprionids or their primary parasitoids (Laing & Heraty, 1981; Heraty & Darling, 1984). In Britain, particular species are known to develop as parasitoids of Diprionidae (Hinks, 1971), Chrysopidae (Clancy, 1946) and Tenthredinidae (Ferriére & Kerrich, 1958), but the possibility of secondary parasitism may not have been thoroughly investigated. A North American species of Perilampus has also been recorded as a hyperparasitoid of immature Orthoptera via Sarcophagidae (Tripp, 1962).
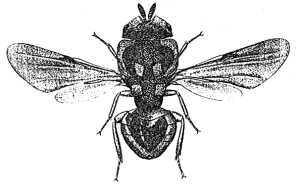
Philomidinae
(Philomides aethiopicus)
Species of Perilampinae are most commonly encountered feeding on flowers, but some feed on aphid honeydew and the female of one species, Perilampus aeneus, has been observed to puncture the epidermis of a leaf and feed on the exudate. The adult female lays up to 500 eggs in the vicinity of the primary host. These eggs, which are suboval in profile and have a short broad peduncle at one end, may be lightly attached to the foliage, or partially embedded in an incision made by the female in a leaf. From this egg emerges the planidial first instar larva. It has sclerotised bands on the first 12 segments, a pair of strong caudal cerci on the 12th segment and the 13th segment is expanded to form a caudal sucker. The sclerotised segmental bands may have sharp teeth along their posterior margins which aid the locomotion of the larva. It also posesses functional spiracles (Heraty & Darling, 1984).
The first instar larva waits for the arrival of a primary host which it boards and generally enters, normally by penetrating the skin. It may search in the haemocoel for the larva of a primary parasitoid, and, in turn, enter this. Further development usually does not occur until the host pupates. When this occurs, the planidial larva exits from the host and feeds externally on the host pupa. There are three or four larval instars; the second instar completely lacks the specialised characters of the first, the third is indistinctly segmented and the fourth very robust, often having a pair of lateral tubercles on each thoracic segment. Pupation takes place in the host cocoon or puparium.
On the other hand, little is known of the biology of species of the other subfamily of Perilampidae, the Chrysolampinae, but the British species Chrysolampus thenae has been studied by Askew (1980). The larva of this species was found to develop as an ectoparasitoid of Meligethes pedicularis (Coleoptera: Nitidulidae) living in the flower heads of betony (Betonica officinalis). Several first instar larvae of the chrysolampine were found attached to each mature nitidulid larva. The parasitoid larvae were usually positioned on the ventral side of the host, attached transversely in the thoracic intersegmental grooves. They remained minute until after the fully-fed host larva had left the flower head and constructed a pupation chamber in the soil. The host was destroyed before it pupated. Only one chrysolampine developed to maturity on each host. The chrysolampine overwintered as a fully-fed larva and pupated the following spring.
The ovarian eggs of C. thenae are elongately fusiform, slightly curved and lack any peduncle. The first instar larva is more or less hymenopteriform, but strongly tapered posteriorly, 13-segmented and bears triangular cup-shaped structures on segments 2-9 (Askew, 1980). The last segment is bilobed. The final instar larva is typically hymenopteriform but unusual in having abdominal spiracles present only on segments 1, 3 and 5. Segments 2, 4 and 6 bear setae.
In North America a species of Chrysolampus has been reared as a parasitoid of Lyctus sp. (Coleoptera: Lyctidae) (Crawford, 1914) and a second species has been reared from wood infested with anobiid beetles (Burks, 1979).
Identification
Ferrière & Kerrich, 1958 (British species); Steffan, 1952; Boucek, 1956 (Central European genera), Boucek (1978) (key to genera).
References
Askew, R.R. 1980. The biology and larval morphology of Chrysolampus thenae (Walker) (Hymenoptera, Pteromalidae). Entomologist's Monthly Magazine 115:155-159.
Burks, B.D. 1979. Torymidae (Agaoninae) and all other families of Chalcidoidea (excluding Encyrtidae):748-749, 768-889, 967-1043.. In: Krombein, K.V.; Hurd, P.D. jr.; Smith, D.R.; Burks, B.D., Editors.) Catalogue of Hymenoptera in America north of Mexico 1 Smithsonian Institute Press, Washington, D.C.
Clancy, D.W. 1946b. The insect parasites of Chrysopidae (Neuroptera). Univ. Calif. Publs Ent. 7:403-496.
Clausen, C.P. 1940. Entomophagous Insects :688pp. McGraw Hill, New York; London.
Crawford, J.C. 1914. The species of Perilampidae of America north of Mexico. Proceedings of the Entomological Society of Washington 16(2):69-76.
Ferrière, C. & Kerrich, G.J. 1958. Hymenoptera 2. Chalcidoidea. Section (a) Agaontidae, Leucospidae, Chalcididae, Eucharitidae, Perilampidae, Cleonymidae and Thysanidae. Handbooks for the Identification of British Insects 8(2)(a):40pp, 79 figs, 5 Plates.
Heraty, J.M. & Darling, D.C. 1984. Comparative morphology of the planidial larvae of Eucharitidae and Perilampidae (Hymenoptera: Chalcidoidea). Systematic Entomology 9(3):309-328.
Hinks, C.F. 1971. Observations on larval behaviour and avoidance of encapsulation of Perilampus hyalinus (Hym., Perilampidae) parasitic in Neodiprion lecontei (Hym., Diprionidae). Canadian Entomologist 103(2):182-187.
Laing, J.E. & Heraty, J.M. 1981. The parasite complex of the overwintering population of Epiblema scudderiana (Lepidoptera: Olethreutidae) in southern Ontario. Proceedings of the Entomological Society of Ontario 112:59-67.
Tripp, H.A. 1962. The biology of Perilampus hyalinus Say (Hymenoptera: Perilampidae), a primary parasite of Neodiprion swainei Midd. (Hymenoptera: Diprionidae) in Quebec, with descriptions of the egg and larval stages. Canadian Entomologist 94(12):1250-1270.
Previous page | Next pageLast updated 07-Jun-2004 Dr B R Pitkin