Universal Chalcidoidea Database
Notes on families
Aphelinidae
Main diagnostic characters
1. Body not metallic (99%)
2. Gaster broadly sessile not narrowed between gaster and propodeum
(100%)
3. About 0.6-1.4mm long (100%)
4. Notaular lines present as deep grooves and straight (100%)
5. Antenna 5-8 segmented
6. Tarsi 4- or 5-segmented (100%)
7. Cerci at apex of gaster
Included taxa
The family currently includes 33 genera and 1168 species placed in 7 subfamilies as follows: Aphelinidae (11/299), Azotinae (1/91), Calesinae (1/3), Coccophaginae (13/699), Eriaphytinae (1/2), Eriaporinae
(5/22), Eretmocerinae (1/52).
 |
 |
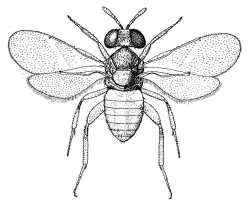
| Aphelininae (Aphelinus abdominalis) |
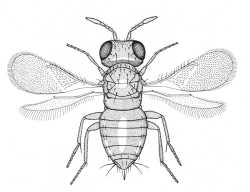
Aphelininae (Aphytis africanus) |
 |
 |
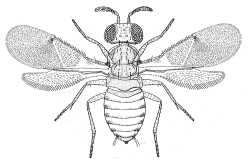
| Aphelininae (Aphytis diaspidis) |
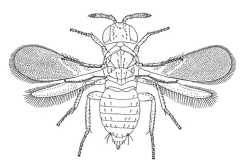
Aphelininae (Centrodora penthimiae) |
Biology
Worldwide, the majority of aphelinids are parasitoids of sternorrhynchous Homoptera. While a few attack species of Aphidoidea, Aleyrodoidea or Psylloidea, most develop by consuming scale-insects (Coccoidea), either as internal parasitoids, as ectoparasitoids (under the scale), or as predators of their eggs. The males of a number of species develop as hyperparasitoids of coccoids via their eulophid, aphelinid or encyrtid primary parasitoids. A number of aphelinids are internal parasitoids of the eggs of various Auchenorrhyncha, Reduvioidea, Lepidoptera or Orthoptera whilst a very few develop on the larvae or pupae of Dryinidae (Hymenoptera), Cecidomyiidae or Chamaemyiidae (Viggiani, 1984). In Britain, a considerable proportion of the aphelinid fauna comprises species that are primary endoparasitoids of aphids (eg Aphelinus spp.).
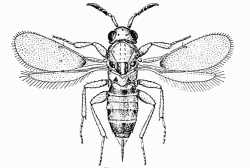 |
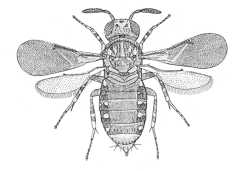 |
| Aphelininae (Centrodora salicis) |
Aphelininae (Marietta connecta) |
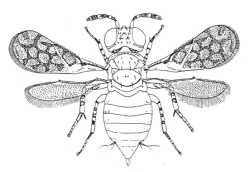 |
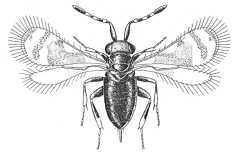 |
| Aphelininae (Marietta leopardina) |
Azotinae (Ablerus mokrzeckii) |
Adult aphelinids may feed on honeydew exuded by their hosts, or on secretions issuing from the wound caused during oviposition. A species of Coccophagus has even been observed to stroke its coccid host to induce it to secrete honeydew (Cendana, 1937). The eggs of aphelinids are often stalked. The first instar larva of ectophagous and some endophagous species is hymenopteriform with four pairs of spiracles, but a number of endoparasitic species have an apneustic caudate primary larva. Larvae of ectoparasitic species (eg Aphytis spp.) have a functional tracheal system and spiracles (Rosen & DeBach, 1979), but those which are endoparasitoids (eg Coccophagus) have larvae with neither spiracles nor a functioning tracheal system (Cendana, 1937). Pupation may take place inside or outside the host. Some species pupate inside the living host within a pupation chamber which becomes filled with air. There is some evidence that air inside this chamber is derived from the hosts tracheal system as in the Encyrtidae (Clausen, 1940). Parasitoids of scales and aphids emerge by cutting a hole through the integument of the host mummy, but if the scale has a delicate covering they push their way out from beneath it. The adults of some such species lack functional mandibles (Viggiani, 1984). Overwintering is normally as a mature larva or pupa.
 |
 |
| Azotinae (Ablerus sp.) |
Calesinae (Cales noacki) |
 |
 |
| Coccophaginae (Coccobius testaceus) |
Coccophaginae (Coccophagus ochraceus) |
The Aphelinidae are very unusual in that the males and females may have different ontogenies. The females of such species always develop as primary endoparasitoids of homopterous hosts (usually coccoids), whilst the males have host relationships that differ from those of conspecific females: they may be primary ectoparasitoids of Homoptera, hyperparasitoids of other chalcidoid larvae or pupae within their homopterous hosts, or primary endoparasitoids of lepidopteran eggs (Flanders, 1937; Walter, 1983a,b). The hyperparasitic males of some species may even develop as facultative or obligate hyperparasitoids on females, and sometimes males, of their own species. Such divergent male ontogeny has been termed heteronomy by Walter (1983).
 |
 |
| Coccophaginae (Coccophagus semicricularis) |
Coccophaginae (Encarsia citrina) |
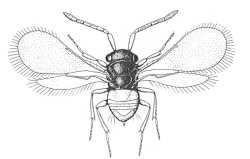 |
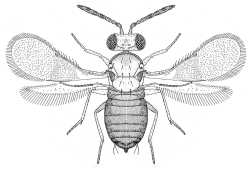 |
| Coccophaginae (Encarsia margaritiventris) |
Coccophaginae (Encarsia perniciosi) |
A variety of classifications have been proposed for different forms of heteronomy (eg Flanders, 1959; 1967; Zinna, 1961; Ferrière, 1965) but that presented here is the simplified system proposed by Walter (1983a). This author recognized three major categories of heteronomous aphelinid parasitoids. Given that the females are, always primary endoparasitoids of Homoptera, these are:
1) Diphagous parasitoids. In these the male is a primary ectoparasitoid of the same host species as the female: both sexes are utilizing the same host but feeding in different ways. An example of a diphagous species is Coccophagus hemera (Flanders, 1959).
2) Heterotrophic parasitoids. In these species (eg Encarsia porteri) the male is an obligate primary endoparasitoid of lepidopteran eggs (Polaszek et al., 1995).
3) Heteronomous hyperparasitoids. These are species in which the males develop as hyperparasitoids of aphelinid, encyrtid, eulophid or platygastrid larvae or pupae. This category can be divided into three:
i) Obligate autoporasitoids where males always develop on conspecific individuals of either sex. Examples of this form of development are Coccophagoides utilis where the male is an ectoparasitoid (Broodryk & Doutt, 1966) and Coccophagus semicircularis in which the male is an endoparasitoid (Zinna, 1961).
ii) Facultative autoparasitoids where males develop either on conspecific individuals, or on other species. Coccophagus lycimnia has ectoparasitic males that are facultative autoparasitoids (Rubin & Beirne, 1975), whilst the African species, C. capensis, is similar but the males are endoparasitoids (Flanders, 1967).
iii) Alloparasitoids where males always develop as parasitoids of non-conspecific chalcidoids. Many examples of this form of development are given by Walter (1983a).
However, as pointed out by Williams and Polaszek (1996) in their review of heteronomous aphelinids, there are no well-documented cases of either true obligate autoparasitism or true alloparasitism. They regard both "obligate" (habitual) autoparasitism and alloparasitism as extremes of a range of facultative responses.
Accompanying the markedly different sexual ontogenies in this group is an exceptional degree of sexual dimorphism in the immature stages. Several species (eg Coccophagus lycimnia) have sexually dimorphic eggs (Flanders, 1937; Viggiani & Mazzone, 1978; Mazzone & Viggiani, 1984) and the larvae of many taxa are unusually sexually dimorphic. For example, the male first instar larva of Coccophagus scutellaris is clothed in long setae and has a spine-like tail, whilst the female larva has no long setae and is of the simple caudate type (Flanders, 1937). The pupae of some species (eg Encarsia spp.) also exhibit sexual dimorphism (Viggiani & Mazzone, 1978).
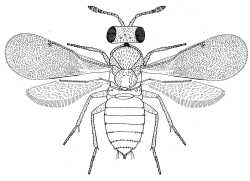 |
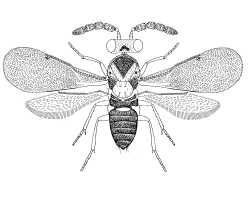 |
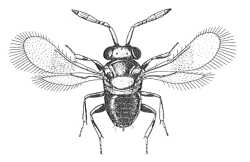
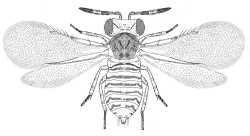
| Female | Male |
| Coccophaginae (Euxanthellus philippiae) |
|
 |
 |
| Coccophaginae (Pteropthrix dimidiata) |
Coccophaginae (Pteroptrix sp.) |
Many species of aphelinids are useful in the biological control of insect pests. Encarsia formosa is used to control Trialeurodes vaporariorum, a serious pest of numerous horticultural plants in greenhouses, and Aphelinus mali successfully controls Eriosoma lanigerum on apple in some areas. Aphytis holoxanthus was introduced into Florida, Mexico, Israel and South Africa from Hong Kong and effectively controlled Chrysomphalus aonidum, a pest of citrus.
 |
 |
| Eretmocerinae (Eretmocerus serius) |
Eriaporinae (Euryischia inopinata) |
 |
|
| Eriaporinae (Promuscidea unifasciativentris) |
|
Identification
Ferrière, 1965 (Mediterranean species including most that occur in Britain); Peck, Boucek & Hoffer, 1964 (Central European genera); Graham, 1976 (British species of Aphelinus), Hayat, 1983 (review of World genera); Hayat, 1998 (Indian genera and species); Huang & Polaszek (Chinese species of Encarsia); Rosen & DeBach 1979 (World Aphytis).
References
Cendana, S.M. 1937. Studies on the biology of Coccophagus (Hymenoptera) a genus parasitic on nondiaspine Coccidae. University of California Publications in Entomology 6(14):337-399.
Clausen, C.P. 1940. Entomophagous Insects :688pp. McGraw Hill, New York; London.
Ferrière, C. 1965. Hymenoptera Aphelinidae d'Europe et du bassin Mediterranean :206pp. Masson et Cie, Paris.
Flanders, S.E. 1937a. Ovipositional instincts and developmental sex differences in the genus Coccophagus. University of California Publications in Entomology 6:401-422.
Flanders, S.E. 1937b. Starvation of developing parasites as an evolution of immunity. Journal of Economic Entomology 30(6):970-971.
Flanders, S.E. 1959. Differential host relations of the sexes in parasitic Hymenoptera. Entomologia Experimentalis et Applicata 2:175-142.
Flanders, S.E. 1967. Deviate ontogenies in the aphelinid male (Hymenoptera) associated with ovipositional behaviour of the parental female. Entomophaga 12(5):415-427.
Graham, M.W.R. de V. 1976. The British species of Aphelinus with notes and descriptions of other European Aphelinidae (Hymenoptera). Systematic Entomology 1(2):123-146.
Hayat, M. 1983. The genera of Aphelinidae (Hymenoptera) of the World. Systematic Entomology 8:63-102.
Hayat, M. 1998. Aphelinidae of India (Hymenoptera: Chalcidoidea): a taxonomic revision. Memoirs on Entomology International
13:viii+416pp.
Huang, J. & Polaszek, A. 1998. A revision of the Chinese species of Encarsia Förster (Hymenoptera: Aphelinidae): parasitoids of whiteflies, scale insects and aphids (Hemiptera: Aleyrodidae, Diaspididae, Aphidoidea). Journal of Natural History 32:1825-1966.
Mazzone, P. & Viggiani, G. 1984. Osservazioni morfo-biologiche sugli stadi preimmaginali di Prococcophagus varius Silv. e P. saissetiae Ann. e Mynh. (Hym. Aphelinidae), parassiti di Saisetia oleae Oliv. .(Hom. Coccoidea). Bolletino del Laboratorio di Entomologia Agraria 'Filippo Silvestri' 41:143-148.
Peck, O. Boucek, Z. & Hoffer, A. 1964. Keys to the Chalcidoidea of Czechoslovakia (Insecta: Hymenoptera). Memoirs of the Entomological Society of Canada No 34:170pp, 289 figs.
Polaszek, A., Stouthamer, R., Hunter, M.S., Rose, M., Ovruski, S.M., Frias, E. & Rojas, S.P. 1994. Heterotrophic parasitoids revisited: preliminary observations on Encarsia porteri (Mercet) (Hymenoptera: Aphelinidae). Colloques de l'INRA No 73:32-32.
Rosen, D. & DeBach, P. 1979. Species of Aphytis of the World (Hymenoptera: Aphelinidae). Series Entomologica 17:801pp.
Viggiani, G. 1984. Bionomics of the Aphelinidae. Annual Review of Entomology 29:257-276.
Viggiani, G.; Mazzone, P. 1978. Morfologia, biologia e utilizzazione di Prospaltella lahorensis How. (Hym. Aphelinidae), parassita esotico introdotto in Italia per la lotta biologica al Dialeurodes citri (Ashm.). Bollettino del Laboratoro di Entomologia Agraria 'Filippo Silvestri' 35:99-161.
Walter, G.H. 1983a. 'Divergent male ontogenies' in male Aphelinidae (Hymenoptera: Chalcidoidea): a simplified classification and a suggested evolutionary sequence. Biological Journal of the Linnean Society 19:63-82.
Walter, G.H. 1983b. Differences in host relationships between male and female heteronomous parasitoids (Aphelinidae: Chalcidoidea): a review of host location, oviposition and pre-imaginal physiology and morphology. Journal of the Entomological Society of Southern Africa 46(2):261-282.
Williams, T. & Polaszek, A. 1996. A re-examination of host relations in the Aphelinidae (Hymenoptera: Chalcidoidea). Biological Journal of the Linnean Society 57:35-45.
Zinna, G. 1961. Specializzazione entomoparassiti negli Aphelinidae: studio morfologico, etologico e fisiologico del Coccophagus bivittatus Compere, nuovo parassita del Coccus hesperidum L. per l'Italia. Bollettino del Laboratorio di Entomologia Agraria 'Filippo Silvestri' 19:302-358
Previous page | Next pageLast updated 05-Sep-2003 Dr B R Pitkin